ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Properties of Objects and Systems Videos 10 videos
AP Physics 2: 1.2 Properties of Objects and Systems. If the amount of mass lost during a nuclear reaction doubles, what happens to the energy relea...
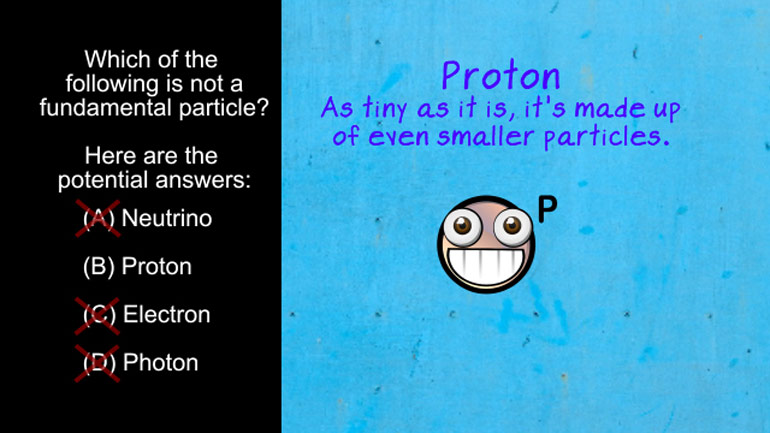
AP Physics 2: 1.3 Properties of Objects and Systems. Which of the following is not a fundamental particle?
AP Physics 2: 2.1 Properties of Objects and Systems. Which of the following pairs of charged conducting spheres would exhibit attraction?
AP Physics 2: 2.4 Properties of Objects and Systems 166 Views
Share It!
Description:
AP Physics 2: 2.4 Properties of Objects and Systems. How could you show the carnival barker an emission spectrum?
Transcript
- 00:00
Hurry And here's your shmoop too sure brought to you
- 00:05
by carnival barkers And no we don't mean dogs that
- 00:08
run the tilt a world machine Yeah Different All right
- 00:12
At the carnival barker offers to give a machine that
- 00:14
makes hydrogen atoms to anyone who can show him any
Full Transcript
- 00:16
mission spectrum How could we do this and win the
- 00:19
machine like let's Make a deal All right And here
- 00:22
the potential answers Interesting That split hired an atlantic good
- 00:28
one All right we've seen the ring toss and the
- 00:30
game where you throw darts at balloons But we've never
- 00:33
seen the emission spectrum game but what's in a mission
- 00:36
spectrum First of all well when energy is added to
- 00:39
an atom the electrons in orbit get all worked up
- 00:43
They jump up into the next orbital energy level Like
- 00:46
us when we get all sugared up Yeah but eventually
- 00:49
they go back down to a lower level When they
- 00:51
go back down to a lower level of energy the
- 00:53
electrons emit a photons will The wavelength of the photon
- 00:56
is determined by the amount of energy lost when it
- 00:59
moves down and that wavelength determines the color of the
- 01:02
light That we see or don't see the wave length
- 01:07
can produce infrared or ultraviolet light that's invisible to us
- 01:11
that the correct answer is a way have to get
- 01:14
those electrons jumping up in order to make them fall
- 01:17
back down in its unexcited state We can't remove energy
- 01:20
from it so b is out and putting two hydrogen
- 01:24
atoms together isn't going to do much Maybe they'll become
- 01:26
friends and hang out together but well that won't produce
- 01:29
any photons so see won't work And if we split
- 01:32
the atom like answer d says well that might make
- 01:35
the whole carnival go Kaboom yeah that's what would happen
- 01:38
so no to get an emission spectrum we have to
- 01:42
get electrons all hopped up so they convention fall back
- 01:45
down So the correct answer is a
Related Videos
AP Physics 2: 1.1 Properties of Objects and Systems. What is the magnitude and direction of the conventional current in this wire?
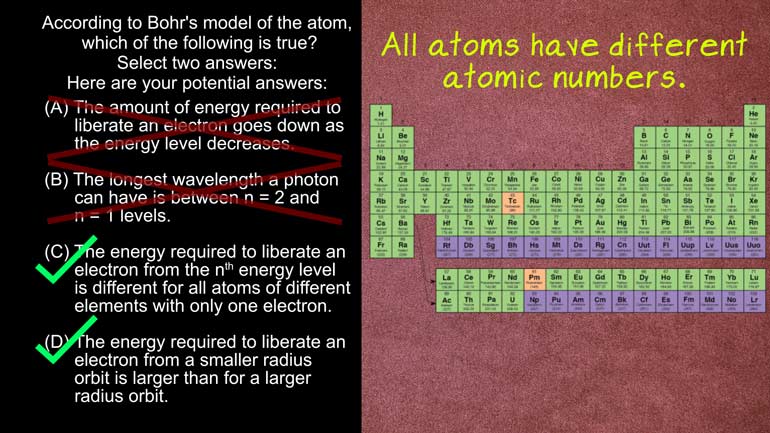
AP Physics 2: 1.5 Properties of Objects and Systems. According to the Bohr's model of the atom, which of the following are true?
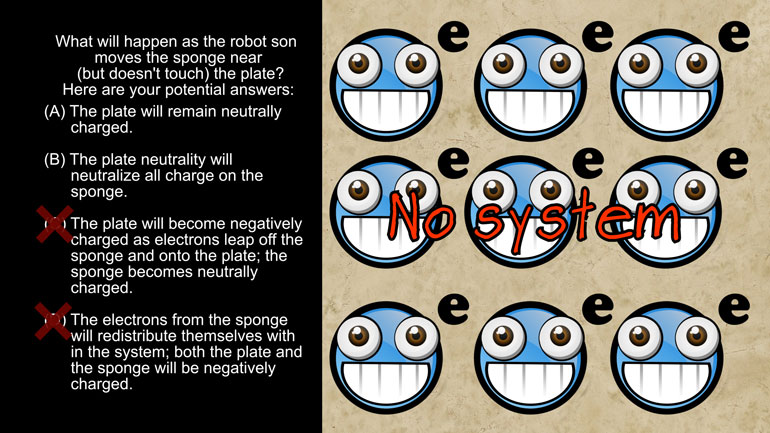
AP Physics 2: 2.2 Properties of Objects and Systems. What will happen as the robot son moves the sponge near (but doesn't touch) the plate?
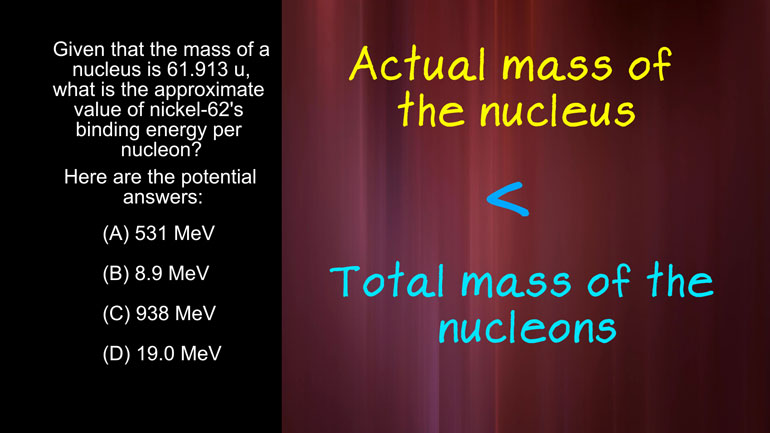
AP Physics 2: 1.4 Object Interaction and Forces. What is the approximate value of nickel-62's binding energy per nucleon?