ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Science Videos 686 videos
In this video, we dive beneath the sea to review the kinds of interesting animals that live in the deep blue.
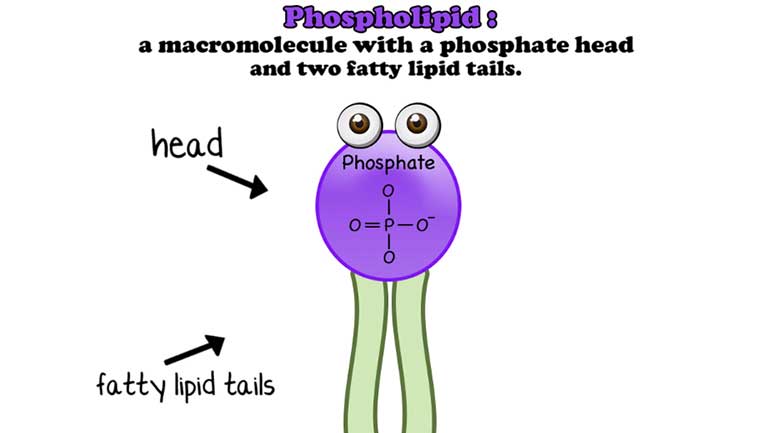
Anything that has a cell (bacteria, listen up!) has phospholipids that keep the cell contained and give it form and shape. Phospholipids protect us...
AP Biology 1.1 Free Energy and Molecular Building Blocks 1116 Views
Share It!
Description:
AP Biology: Free Energy and Molecular Building Blocks Drill 1, Problem 1. Which statement incorrectly describes the properties of water?
Transcript
- 00:03
Here's your shmoop du jour...a la Bio.
- 00:07
Which statement incorrectly describes the properties of water?
- 00:13
And here are the potential answers...
- 00:18
Water. H20. That stuff we're all supposed to drink 8 glasses of every day.
- 00:23
Even those of us with a drinking problem.
Full Transcript
- 00:27
But aside from hydrating our body, it has some other really cool, unique properties
- 00:30
that are good to know for the AP bio test. And since this is a "which statement incorrectly
- 00:36
describes" question...
- 00:37
...we can just look at every single answer choice to determine which one ain't doin'
- 00:42
it for us. Starting with our first answer choice...is
- 00:45
water a nonpolar molecule? Well, what does that mean? Does it just like
- 00:50
the thermostat cranked up to 90? Nope... polarity in science refers to differences
- 00:55
in charge or ELECTRONEGATIVITY between atoms in a molecule.
- 01:02
Electronegativity means the tendency to attract electrons. With or without makeup.
- 01:08
In this problem, it means that if our water molecule were to have a different electronegativity
- 01:13
around oxygen compared to its two hydrogen atoms, the molecule would be polar.
- 01:18
Hm ok...so we know that Oxygen is a super electronegative atom, and attracts lots of
- 01:25
negative charge to it. It's something we should just know and memorize
- 01:30
because it's one of those things that is just uber-important.
- 01:33
Hydrogen, on the other hand, does not have a high electronegativity. Sorry, hydrogen.
- 01:38
We're sure you make up for it by having a great personality.
- 01:43
So even though there are two hydrogen atoms for one oxygen in every water molecule...
- 01:48
...the water molecule is still super negative around the oxygen and is unbalanced in charge.
- 01:54
This difference in charge makes water POLAR. So our answer is A.
- 01:58
Now start chugging. You've still got 6 glasses to go today.
Related Videos
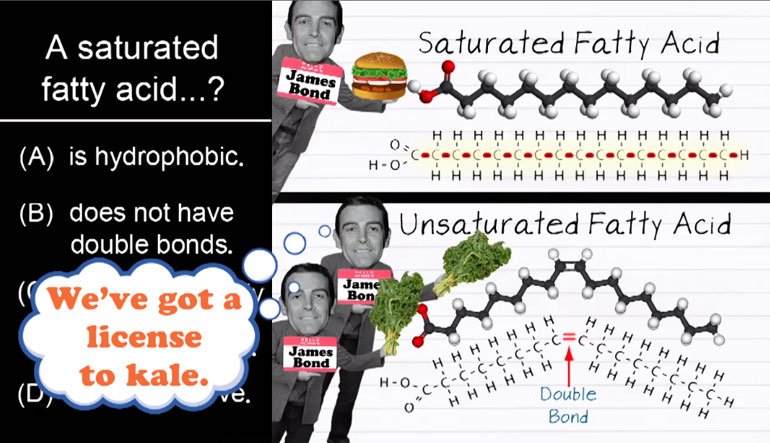
AP Biology: Biological System Interactions Drill 1, Problem 1. Complete the sentence about a saturated fatty acid.
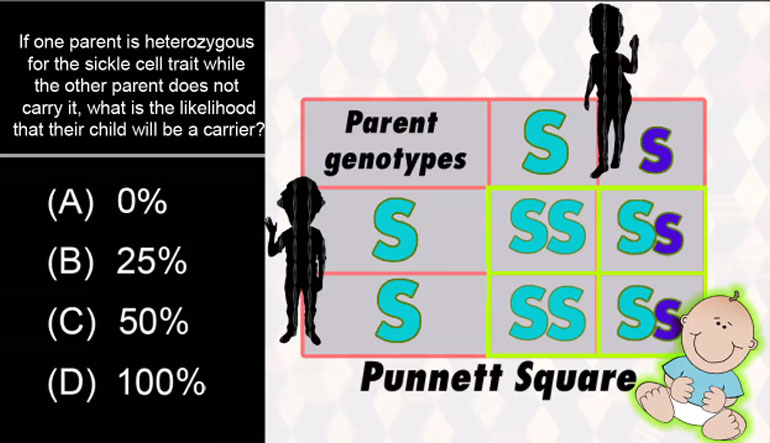
AP Biology: Essential Life Process Information Drill 1, Problem 1. If one parent is heterozygous for the sickle cell trait while the other par...
AP Biology: Evolution Drives the Diversity and Unity of Life Drill 1, Problem 1. The first cells on planet Earth were likely what?
AP® Biology: Evolution Drives the Diversity and Unity of Life Drill 1, Problem 2. What was likely the first genetic material?
AP Biology: Evolution Drives the Diversity and Unity of Life Drill 1, Problem 4. Hardy-Weinberg equilibrium requires that a population meet al...